“Cancer cells often overlook the stop signs”
Freiburg, Mar 14, 2019
Like every other cell type, immune cells need food. Dr. Julia Jellusova at the Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies examines how they respond to starvation and surplus. Metabolism influences the function of these cells, and it may even contribute to their becoming cancerous. Jellusova, an immunologist, aims to find out more about this - and maybe how to put the brakes on lymphoma.
 Julia Jellusova has loved biology since childhood – with Metabolic Signalling she is working in a new and promising field of research.
Julia Jellusova has loved biology since childhood – with Metabolic Signalling she is working in a new and promising field of research.
Photo: Harald Neumann
In order to fight many pathogens such as harmful bacteria, the immune system often needs antibodies. But a number of things have to happen before antibodies can be produced. Special immune cells – B cells – meet the pathogen, start to divide, and go through an internal remodeling process and finally produce antibodies. This takes a lot of energy. “The right environment has to be there for these processes to happen,” says Dr. Julia Jellusova of BIOSS and CIBSS, the University of Freiburg’s Clusters of Excellence in the field of biological signalling research. B cells that do not get enough oxygen and nutrients cannot mature properly. “Just how immune cells respond to their environment and regulate their metabolism is a new and exciting topic in immunology,” says Jellusova. Altered nutrient status and defects in metabolism may be important for the development of cancer and autoimmune disorders.
Division happens too fast
“Immunologists always thought that B cells just take what they need,” Jellusova explains. Far from it. B cells respond differently if their environment is not right – if there is a lack of nutrients or oxygen, or if their metabolism is not running smoothly, or if certain regulators fail. The 34-year-old researcher has already investigated one of these regulators: the metabolic brake GSK3. The maturation process of B cells includes strong phases of rapid division. For this exhausting act, the metabolism of these cells must be boosted so that the cells can take in and process more nutrients. But metabolism and cell division should not run too fast – otherwise these processes may get out of control. “GSK3 says to the cell: you are dividing too quickly,” Jellusova says. Then the B cells take in less food, turn down their metabolism and stop dividing.
|
Biological signals and signalling pathways Precise coordination is needed in order for trillions of cells to form, maintain and regenerate orderly tissues, organs and healthy organisms. To achieve this, complex communications processes take place between and within cells. Defects in these communications networks can lead to developmental disorders, immunodeficiency, cancer and other diseases. The CIBSS Cluster aims to achieve a comprehensive understanding of these communications processes – from the molecular level to the level of cells and organs. Furthermore, CIBSS research groups will investigate how these communications processes are connected to other important biological processes such as metabolism. Based on these new findings, the researchers will develop new possibilities to address challenges in areas as diverse as immunotherapy and the sustainable production of crops.
|
|
“I switched off GSK3 genetically,” says Jellusova. In comparison to normal B cells, those with inactive GSK3 divide faster if there is enough food. But they die much sooner if they get hungry. “The cells don’t know when they need to stop reproducing,” she hypothesizes. Sooner or later they run out of nutrients and energy and cells starve. Jellusova wants to answer a number of questions, such as: How exactly does GSK3 work? Why do B cells without GSK3 degenerate and become cancer cells? “Cancer cells often overlook the stop signs,” Jellusova explains. They divide without restraint, and they use up enormous amounts of nutrients to do so. That means cancer cells have to be highly resistant to hunger. Sometimes another protein helps. “It sends survival signals.” And this protein is frequently active in the signal pathways of degenerate B cells. Maybe it even pushes starving B cells with inactive GSK3 to become cancer cells?
Along with research - children’s books
Jellusova’s special field, metabolic signalling, is an important new research area in CIBSS. She has been interested by the topic of immune cells and nutrients since she wrote her diploma thesis, and biology has been her fascination since childhood. “Even when I was five, I dried plants and made a herbarium,” she says. Her father gave her a microscope for her sixth birthday. “It was my greatest treasure.” Later she studied Biology in Erlangen, where she completed her doctorate in Immunology in 2010. After a stint as a postdoc in La Jolla, California, she came to Freiburg in 2016. “Freiburg is exceptional in the field of metabolism and the immune system. There is hardly any other place with expertise like we have here.” Sponsored by the Margarete von Wrangell habilitation program, Jellusova heads a three-person working group in the BIOSS Signalhaus. On the side, she writes children’s books, most recently “Entdecke dein Immunsystem” (Discover your immune system).
Jellusova plans to investigate a certain protein: p62, also known as sequestosome 1. “It is an adaptor protein that regulates signalling in B cells by bringing different molecules together.” If there is not enough food, B cells can recycle old mitochondria and other parts of their own cell. By using up superfluous or damaged parts of the cell, B cells can meet their own energy needs for a time. “p62 plays a role in this. But nobody knows what happens when p62 doesn’t work.” Jellusova will first switch off the protein in normal B cells, then in hungry ones. What does p62 do in these situations? Then, using peptides and other molecules, she aims to inhibit, stimulate different steps in the p62 signalling pathway in B cells. She is especially interested in finding out whether p62 can contribute to the degeneration of B cells into cancer cells. “In other cell types p62 can go either way - it can promote or inhibit the formation of cancer cells.”
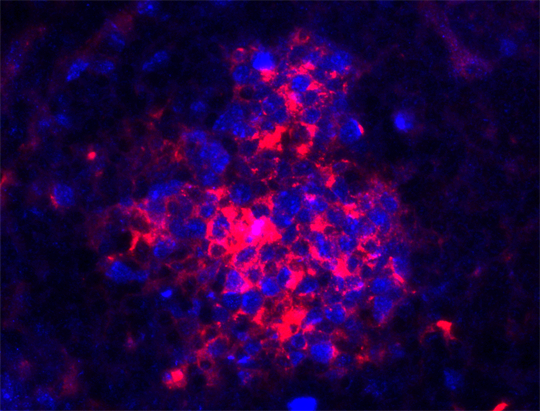 Lymphoma mostly develops from B cells (red). Source: Julia Jellusova
Lymphoma mostly develops from B cells (red). Source: Julia Jellusova
Does metabolism contribute to autoimmune disorders?
How do lymphomas arise? “Lymphoma usually develops from B cells,” Jellusova explains. These cells degenerate quite often because they have several rapid division phases in their maturation process and they also mutate. After pathogen encounter, mutation and division lead to the production of many unique B cells. Each one has on its cell surface a different receptor antibody-like receptor for recognition of the pathogen. “But there are good and bad receptors. B cells with bad receptors die.” After this tough selection process, the best B cells remains – those that recognize the pathogen but don’t attack the body’s own cells. These B cells further divide and their successors produce the antibodies which normally help the immune system to defeat the pathogen.
But how do B cells respond to metabolic stress, and how does it influence their function? These are the issues that are currently tantalizing Julia Jellusova. Some things are already known, for instance, that B cells have internal receptors for certain nutrients, such as glucose and amino acids. “These receptors in the cell measure how much of these nutrients are present.” But beyond that, there are many gaps in knowledge. What role does metabolism play when B cells develop into cancer cells or in B cell-driven autoimmune disorders such as lupus erythematodes and rheumatoid arthritis? At the moment Jellusova is concentrating on basic research – particularly on the function of the p62 protein. “But I hope that at some stage this research will lead to new approaches to treat cancer and or to stop autoimmune disorders in their tracks.”
Jürgen Schickinger
CIBSS – Centre for Integrative Biological Signalling Studies
CIBSS ist einer der beiden neuen Exzellenzcluster der Universität Freiburg. Darin werden mehr als 60 Arbeitsgruppen biologische Signalprozesse von Immunzellen, rund um Mitochondrien, in der Organentwicklung und in Wurzeln von Pflanzen erforschen. Die Wissenschaftlerinnen und Wissenschaftler kommen aus sechs Fakultäten – der Fakultät für Biologie, Mathematik und Physik, Medizin, Chemie und Pharmazie, Technik sowie der Fakultät für Rechtswissenschaften. Auch das Universitätsklinikum sowie das Max-Planck-Institut für Immunbiologie und Epigenetik sind beteiligt. Die Deutsche Forschungsgemeinschaft fördert den Cluster von Anfang 2019 bis Ende 2025.
CIBSS – Centre for Integrative Biological Signalling Studies