The Complicated Search for Mistakes in the DNA Base Sequence
Freiburg, Oct 04, 2019
There are roughly 6,000 to 8,000 rare diseases known today. Many of these are caused by a genetic defect. A disease is considered rare when only one out of 2,000 people is affected. Although it is extremely rare for a person to be diagnosed with any one of these diseases, roughly 5-7 % of the population – about 4 million people in Germany and 30 million in Europe – has a rare disease. This means that these extremely few cases are spread out over great distances, making studies very difficult and diagnoses often only possible after a long ordeal. Meanwhile, experts who research rare diseases are also few and far between. One of these experts is Dr. Miriam Schmidts. She has been working in the Pediatric Genetics team at the Center for Pediatrics of the University Medical Center Freiburg since 2015.
 In the laboratory, Miriam Schmidts is working to identify the genetic defect that causes a rare disease. Photo: Jürgen Gocke
In the laboratory, Miriam Schmidts is working to identify the genetic defect that causes a rare disease. Photo: Jürgen Gocke
Miriam Schmidts has just come from consulting with the parents of one of her patients, a boy who has ciliopathy, a genetic disorder. She has a few minutes to tell us about her research and opens her laptop. The colorful animations that flicker across the screen seem as if they were made for the kids who inspire her research. Hundreds of small hair-like structures on the cell surface called cilia move in sync, like a group of cheerleaders at a pep rally. Two greenish yellow ovals dance on their tips in gentle waves, moving as if on a conveyer belt toward the edge of the screen and out of the picture.
“That’s the way it should be,” Schmidts says. “When the cilia work like they’re supposed to, they transport mucous out of the lungs. When they don’t, the mucous builds up and blocks the lungs.” This is illustrated in the next film. Here, the greenish yellow blobs wiggle more or less in place because the cilia don’t seem to know what direction they’re moving, or even if they’re supposed to do anything at all. But if the mucous is not transported out of the lungs, it can’t be coughed out. Then the lungs start to clog, bacteria collects, and infection sets in – just like with cystic fibrosis – often causing long-term damage.
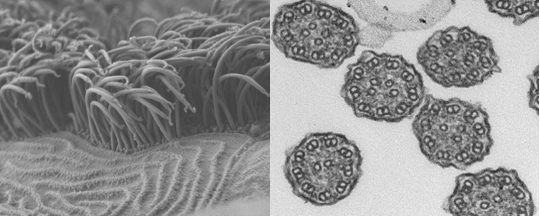 Motile cilia under an electron microscope: whole (left) and cross-section. The job of these ultrafine hair-like structures is to transport mucous out of the lungs. Source: Miriam Schmidts
Motile cilia under an electron microscope: whole (left) and cross-section. The job of these ultrafine hair-like structures is to transport mucous out of the lungs. Source: Miriam Schmidts
The Result of Various Genetic Defects
This dysfunction of the cilia is the result of various genetic defects, explains Schmidts. The small hair-like structures that grow on cells individually or in bushels, depending on the type and the body part, were first discovered under a microscope in 1800. At first, it was believed that they had no function, until it was found out that those that move (motile cilia) are responsible for transporting organic material from A to B: secretions out of the bronchial system, eggs from the uterus, and sperm into the fallopian tube. Non-motile cilia (cilia that do not move), on the other hand, act like antenna on the cell’s surface. Because they are important for processing the cell’s various signaling pathways, non-motile cilia dysfunctions often lead to developmental disorders in various organs, such as the kidneys, the brain, or the skeletal system. From an evolutionary point of view, cilia stem from the flagella found in algae and bacteria.
After Schmidts completed her doctoral studies under the nephrologist Prof. Dr. Gerd Walz at the University Medical Center Freiburg and earned her PhD in 2005, she became a medical resident at the hospital’s Center for Pediatrics. At that time, she worked on a research project on motile cilia under the supervision of Prof. Dr. Heymut Omran, who is now the director of the University Children’s Hospital Muenster. After this, she spent several years at the University College in London, where she researched non-motile cilia, which are thought to function as signal receivers for cells. Genetic defects in non-motile cilia are also responsible for rare diseases. In these cases, children are often born with brain deficiencies, or with more than five fingers on each hand, because certain signaling pathways that are vital for the development of the fingers are impaired.
Schmidts continues to conduct her primary research in this field today. She especially focuses on types of skeletal dysplasia, such as the rare Ellis van Creveld syndrome. In this prenatal anomaly, the growth of the ribs is impaired. Children with this disease are born with ribcages that are much too small, meaning they cannot get enough air and often die before they reach the age of two because there is nothing doctors can do. If they survive, they develop cysts on their kidneys, which leads to kidney failure, or they become blind because the photoreceptors in their eyes stop functioning.
 Miriam Schmidts has been researching at the Clinic for Pediatrics at the University Medical Center Freiburg since 2015. Photo: Britt Schilling/University Medical Center Freiburg
Miriam Schmidts has been researching at the Clinic for Pediatrics at the University Medical Center Freiburg since 2015. Photo: Britt Schilling/University Medical Center Freiburg
A Disease Model Based on Healthy Cells
“In the lab, we’re trying to identify exactly what genetic defect causes these symptoms,” Schmidts says. “That's why we’re using the latest sequencing technologies to find the defective gene causing this disease.” This search is much easier today than even only a few years ago, because scientists can now compare the samples in question with those in the gene databases that contain the base sequences of tens of thousands of healthy people. “What we’re interested in is how the gene causes the defect in the body. And, why does the body develop all these problems, from brain malformation to blindness, if that gene is missing?” In the lab, Schmidts relies on patients’ cell samples as a basis for creating a disease model out of healthy cells in which she then uses the CRISPR/CAS method to recreate the genetic defect found in the patient.
Although Schmidts believes this method could theoretically be used to cure or correct genetic defects in the future by eradicating mistakes in the base sequence, she also believes that this is highly problematic for ethical and medical reasons. “After all, we’re talking about making a change in human DNA and not being able to predict the side-effects and long-term consequences in any way.” One of the main goals of her basic research is therefore to develop new ways of treating these rare diseases that also go beyond gene therapy. “If we were able to manipulate certain signaling pathways in cells using pharmacological means, this could mean considerable improvements for patients.” Schmidts and her team are currently using algae, which have flagella that have the same cilia structure found in humans, to test many authorized substances for their possible, hitherto unknown effects on cilia.
Contact with Patients and Their Families
Miriam Schmidts is a young mother herself, and she values the contact with her young patients and their families just as much as her research: “As a doctor, I want the parents to understand why their child is the way he or she is. This is very important because genetic diseases often mean a huge psychological strain for parents, especially because we can’t cure these diseases and they’re often fatal. Many of them wonder what they’ve wrong. It helps to be able to tell them that the disease couldn’t have been avoided.” She also helps parents to assess the risk of another child developing the same disease.
The phone rings. Miriam Schmidts closes her laptop and excuses herself, she has to take the call. Her patients and their families have her full attention at all times.
Dietrich Roeschmann

